Unveiling the Magic Behind Battery Power: A Deep Dive into Charging and Discharging
Posted: Mon Feb 26, 2024 5:47 pm
In the heart of our electronic devices, from the smartphones in our pockets to the electric cars on our roads, lies a technology fundamental to modern life: the rechargeable battery. Among these, lithium-ion batteries stand out for their efficiency and capacity. But how do these powerhouses store and release energy? Let's unravel this electrochemical marvel, focusing on the intricate dance of lithium ions between the cathode and anode.
The Charging Symphony Begins
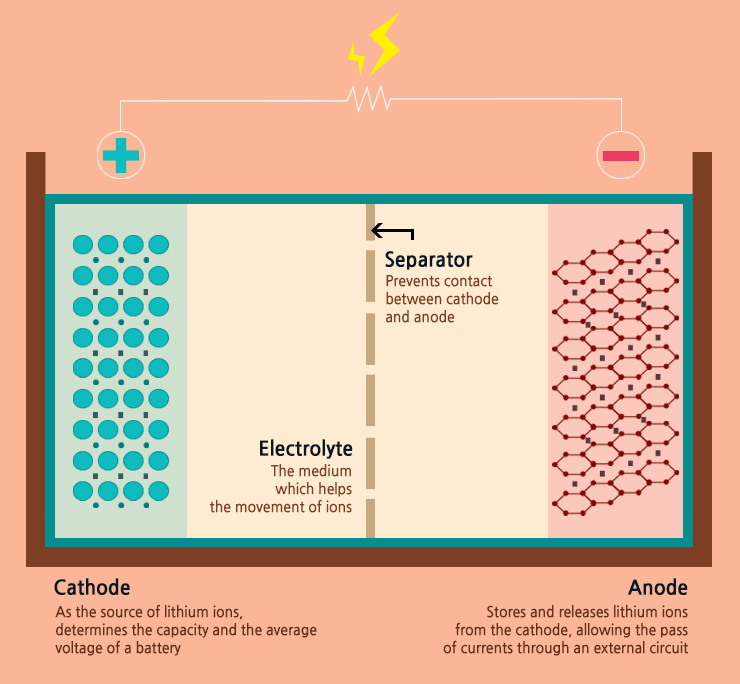
Imagine the battery as a crowded dance floor, with lithium ions and electrons as the dancers. During charging, the music starts, and the lithium ions move gracefully from the Cathode, a lithium metal oxide stage, to the Anode, which can be made of various materials like graphite or the promising silicon carbon, which has been recently explored in smartphones and EVs as well.
The electrolyte, a lithium salt solution, acts as the floor, guiding the ions' path, while a separator ensures they don't bump into each other too directly, preventing a short circuit. As the ions make their way to the anode, electrons flow through the external circuit, injecting energy into the battery.
At the anode, lithium ions find their seats between the material's layers, storing energy through a process known as intercalation. This moment captures the essence of charging: converting electrical energy from an external source into chemical energy within the battery.
The Discharge: Releasing the Energy
When the device is powered on, the ions are called back to the cathode, retracing their steps across the electrolyte dance floor. As they move, electrons flow in the opposite direction through the device, powering it with their energy.
At the cathode, lithium ions are welcomed back, releasing the energy they carried as they de-intercalate from the anode material. This release of energy powers our devices, lighting up screens and spinning motors in a harmonious discharge of power.
The Science Behind the Scenes
The entire process hinges on redox reactions, where lithium ions oscillate between being oxidized and reduced, shedding and gaining electrons. The force propelling the ions back and forth is the electrochemical potential, a difference in energy that drives the ions from high to low potential areas.
But, like any good performance, efficiency is key. Not all energy input during charging makes its way back during discharge. Some is lost as heat, a measure captured by the battery's Coulombic efficiency. Moreover, with each charge and discharge cycle, the battery materials wear slightly, reducing their ability to hold charge over time.
The Cast of Anode Materials
While the cathode often takes center stage with its lithium metal oxide composition, the anode's choice of materials, from graphite to silicon carbon, plays a crucial role in the battery's performance. Each material offers a unique blend of capacity, stability, and efficiency, influencing the battery's overall energy density and lifespan.
The Future of Batteries
As we push the boundaries of technology, understanding the fundamental processes of charging and discharging helps in crafting better batteries. Innovations in materials and design promise batteries that charge faster, last longer, and power our future towards a more sustainable, electrified world.
In the intricate ballet of lithium ions, every movement counts, from the initial charge to the final discharge, powering our world one electron at a time. Join us as we continue to explore and harness the incredible potential of batteries, the silent sentinels of our electronic age.